a. What can change the value of Kc
Only temperature can alter the value of Kc for a reaction because the equilibrium constant Kc has a fixed value for a particular reaction at a specified temperature.
b. The reaction must be at equilibrium for the value of Kc to be calculated.
c. Define the term homogeneous
The reactants and products are all in the same state.
d. What is the rule to determine the units for Kc?
When the number of moles of the products is equal to the reactants, there is no unit for Kc.
(c+d) - (a+b) = 0 no units
(c+d) - (a+b) = 1 mol dm3
(c+d) - (a+b)= -1 mol-1 dm3


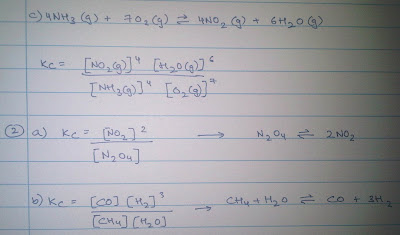
No comments:
Post a Comment