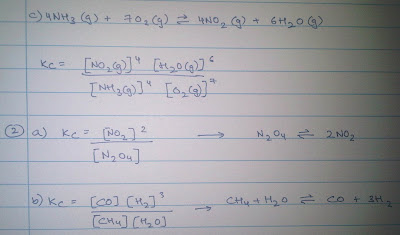Deduce the extent of a reaction from the magnitude of the equilibrium constant.
1. What does the word magnitude mean?
Magnitude is the size.
2. Explain why the three reactions above do not have units for Kc
The three reactions above do not have any units for Kc because the moles of the product is equal to the moles of the reactants.
E.g. 1+1=2
3. Deduce the extent of the reaction if Kc is
- Kc >>1 then the reaction is considered to go almost to completion
- Kc lies between 0.01 and 100 (10-2 and 102), both the products and the reactants are present in sufficient amounts.
- Kc <<1 the reaction hardly proceeds.
Monday, May 23, 2011
7.2.1
Deduce the equilibrium constant expression (Kc) from the equation for a homogeneous reaction.
a. What can change the value of Kc
Only temperature can alter the value of Kc for a reaction because the equilibrium constant Kc has a fixed value for a particular reaction at a specified temperature.
b. The reaction must be at equilibrium for the value of Kc to be calculated.
c. Define the term homogeneous
The reactants and products are all in the same state.
a. What can change the value of Kc
Only temperature can alter the value of Kc for a reaction because the equilibrium constant Kc has a fixed value for a particular reaction at a specified temperature.
b. The reaction must be at equilibrium for the value of Kc to be calculated.
c. Define the term homogeneous
The reactants and products are all in the same state.
d. What is the rule to determine the units for Kc?
When the number of moles of the products is equal to the reactants, there is no unit for Kc.
(c+d) - (a+b) = 0 no units
(c+d) - (a+b) = 1 mol dm3
(c+d) - (a+b)= -1 mol-1 dm3
Sunday, May 15, 2011
7.1.1
Outline the characteristics of chemical and physical systems in a state of equilibrium.
Definition of Equilibrium:
At equilibrium, the rate of the forward reaction is equal to the rate of the backward reaction.
Physical Systems:
An example of this is bromine is placed in a sealed container at room temperature. A layer of bromine gas is formed above the liquid bromine. It occurs in both forms because, due to its boiling point being close to room temperature. Hence some particles have more kinetic energy in order to be in a gas form (evaporation), however when these gas particles collide with the surface of the liquid they lose energy and turn back into liquid (condensation).
Equilibrium is only reached when rate of evaporation is equal to the rate of condensation.
Chemical Systems:
In the reaction of dissociation between hydrogen iodide with H2 and I2, a purple iodine gas is released and the colour increases. However, after a short amount of time colour stops’ increasing indicating equilibrium is reached.
Heating CuSO4 Crystals:
When heating blue copper sulphate (CuSO4) crystals we are converting hydrated CuSO4 crystals to anhydrous CuSO4 crystals. The colour of the crystals changes from blue to white. They have dynamic equilibrium when hydrated crystals are left unconcealed as they lose water due to evaporation however the water vapour condenses back into the crystals; therefore it is a reversible reaction.
Definition of Equilibrium:
At equilibrium, the rate of the forward reaction is equal to the rate of the backward reaction.
Physical Systems:
An example of this is bromine is placed in a sealed container at room temperature. A layer of bromine gas is formed above the liquid bromine. It occurs in both forms because, due to its boiling point being close to room temperature. Hence some particles have more kinetic energy in order to be in a gas form (evaporation), however when these gas particles collide with the surface of the liquid they lose energy and turn back into liquid (condensation).
Equilibrium is only reached when rate of evaporation is equal to the rate of condensation.
Chemical Systems:
In the reaction of dissociation between hydrogen iodide with H2 and I2, a purple iodine gas is released and the colour increases. However, after a short amount of time colour stops’ increasing indicating equilibrium is reached.
Heating CuSO4 Crystals:
When heating blue copper sulphate (CuSO4) crystals we are converting hydrated CuSO4 crystals to anhydrous CuSO4 crystals. The colour of the crystals changes from blue to white. They have dynamic equilibrium when hydrated crystals are left unconcealed as they lose water due to evaporation however the water vapour condenses back into the crystals; therefore it is a reversible reaction.
 |
| Hydrated Copper Sulphate Crystal (blue) |
 |
| Heating Copper Sulphate Crystal |
 |
| Anhydrous Copper Sulphate (white) |
Sunday, May 8, 2011
Practice Questions
1. D-if water is produced it is a neutralisation reaction
2. A
3. D-mol dm-3 time-3
4. B
5. C-catalyst reduces activation energy by taking an alternative route.
6. B
7. C
11. A) measure mass of reactants (g) on top pan balance
Volume of CO2 produced (cm3),
pH, measured using pH probe, of solution (time it takes to reach 7)
B) Increase in temperature means increase in average kinetic energy of particles hence more frequent collision. Higher number of particles with average kinetic energy>Ea.
Increase in surface area of MgCO3 hence more particles on the surface for successful collisions.
Adding a catalyst-alternative route provided with lower activation energy hence more particles with average kinetic energy>Ea therefore more collisions.
C) i) Stay the same because MgCO3 is already in excess. HCl is the limiting factor.
ii) Stay the same because mass of reactants as well as the concentration stays the same. Only reaction takes place faster because higher collision frequency.
2. A
3. D-mol dm-3 time-3
4. B
5. C-catalyst reduces activation energy by taking an alternative route.
6. B
7. C
11. A) measure mass of reactants (g) on top pan balance
Volume of CO2 produced (cm3),
pH, measured using pH probe, of solution (time it takes to reach 7)
B) Increase in temperature means increase in average kinetic energy of particles hence more frequent collision. Higher number of particles with average kinetic energy>Ea.
Increase in surface area of MgCO3 hence more particles on the surface for successful collisions.
Adding a catalyst-alternative route provided with lower activation energy hence more particles with average kinetic energy>Ea therefore more collisions.
C) i) Stay the same because MgCO3 is already in excess. HCl is the limiting factor.
ii) Stay the same because mass of reactants as well as the concentration stays the same. Only reaction takes place faster because higher collision frequency.
6.2.7
Sketch and explain Maxwell–Boltzmann curves for reactions with and without catalysts.
The graph on the right shows the number of particles with energy greater than the activation energy. Catalysed reactions have more of these particles because the route taken has a significantly lower activation energy hence more particles fall under the category: Energy of particles > Ea. It increases the proportion of particles which are able to react. |
6.2.6
Describe the effect of a catalyst on a chemical reaction.
A catalyst is a substance which alters the rate of reaction without being used up. It can increase the rate as well as decrease it. Substances which decrease rate of reactions are called inhibitors. Catalysts use alternative routes for the reaction to take place, with lower activation energy.
A catalyst is a substance which alters the rate of reaction without being used up. It can increase the rate as well as decrease it. Substances which decrease rate of reactions are called inhibitors. Catalysts use alternative routes for the reaction to take place, with lower activation energy.
6.2.5
Sketch and explain qualitatively the Maxwell–Boltzmann energy distribution curve for a fixed amount of gas at different temperatures and its consequences for changes in reaction rate.
Since temperature is a measure of average kinetic energy of the particles, the increase in temperature leads to an overall increase in the rate of reaction. Therefore a greater number of particles will have energy above the activation energy.
The area under both curves is equal (signifying the number of particles) but the curve at 310K has slightly more particles with energy greater than the activation energy. This is because at higher temperatures particles have an increase in average kinetic energy, which means higher collision frequency. Therefore more successful collisions taking place hence increase in rate of reaction.
Since temperature is a measure of average kinetic energy of the particles, the increase in temperature leads to an overall increase in the rate of reaction. Therefore a greater number of particles will have energy above the activation energy.
The area under both curves is equal (signifying the number of particles) but the curve at 310K has slightly more particles with energy greater than the activation energy. This is because at higher temperatures particles have an increase in average kinetic energy, which means higher collision frequency. Therefore more successful collisions taking place hence increase in rate of reaction.
Thursday, April 28, 2011
6.2.4
Predict and explain, using the collision theory, the qualitative effects of particle size, temperature, concentration and pressure on the rate of a reaction.
Questions | Answers |
Independent Variable | Surface area of CaCO3 chips (i.e. powder, chips #2 etc.) |
Dependent Variable | Volume of CO2 produced |
Controlled Variables | Volume of HCl, mass of CaCO3, stop watch, inverted measuring cylinder, top pan balance. |
Using collision theory explain the following shape of the graphs at the start of the reaction. | The larger the surface area of CaCO3 the faster the rate of reaction. This is because in a solid only the particles on the surface can collide successfully hence if it is powdered more particles can come into contact with the other reactant. |
What does the gradient of the graph at any one point represent? | Rate of reaction |
What are the units for the gradient of the graph? | cm3/seconds |
Discuss the reasons for the differences in the shape of the graphs. | The larger the surface area of CaCO3 the steeper the graph should be, due to an increase in rate of reaction. |
Wednesday, April 27, 2011
6.2.3
Describe the collision theory.
Factors affecting rate of reaction | Description | Diagram |
Collision Frequency | Higher the frequency of collision, the higher the probability of successful collision. | |
Collision Geometry | Due to particles coming in different orientations it is necessary for the collision to take place successfully. | |
Number of particles with E ≥ Ea | In order for collision to take place particles must have a minimum amount of energy to overcome repulsion between molecules and to break some bonds in the reactants. Only particles with E ≥ Ea will successfully collide. |
6.2.2
Define the term activation energy, Ea.
The minimum value of kinetic energy which particles must have before they are able to react.
The minimum value of kinetic energy which particles must have before they are able to react.
6.2.1
Describe the kinetic theory in terms of the movement of particles whose average energy is proportional to temperature in Kelvin’s.
The kinetic theory states that when temperature of a substance is increased the average kinetic energy of the particles increases too. However if the temperature reaches absolute zero it means there is no average kinetic energy as there is no motion of particles.
In the apparatus shown below, the increase in voltage mimics the rise in temperature while the height of the polystyrene cylinders represents the increase in volume.
The kinetic theory states that when temperature of a substance is increased the average kinetic energy of the particles increases too. However if the temperature reaches absolute zero it means there is no average kinetic energy as there is no motion of particles.
In the apparatus shown below, the increase in voltage mimics the rise in temperature while the height of the polystyrene cylinders represents the increase in volume.
Monday, April 25, 2011
6.1.2
Suitable experimental procedures for measuring rates of reactions.
6.1.1
Define the term rate of reaction.
The decrease in the concentration of reactants per unit time or the increase in the concentration of product per unit time.
The decrease in the concentration of reactants per unit time or the increase in the concentration of product per unit time.
Sunday, April 3, 2011
Subscribe to:
Comments (Atom)